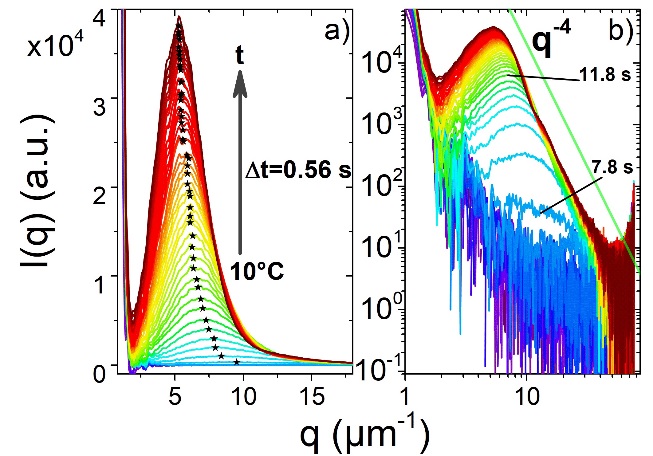
Figure 1: Typical USAXS profiles of an IgG-PEG system during a temperature quench from 38 °C to 10 °C. The correlation peak of spinodal decomposition appears within about 8s, which grows in intensity and shifts its position to lower q values with time.
Phase transitions in biological systems, such as protein crystallization and protein condensation phenomena, are of fundamental importance. They are, for example, of relevance in diseases where protein aggregation is driven by subtle changes in the protein structure and the effective protein-protein interactions. Liquid-liquid phase separation (LLPS) in protein solutions is dominated by both kinetic and dynamic effects in which the separation and role of the two is not always easy to distinguish, in fact, often they may be intimately intertwined [1]. In systems dominated by short-ranged attraction the LLPS is often hindered by the formation of a highly dense phase cutting the gelation line [1-3]. While the early phase of the LLPS is then dominated by a spatio-temporal evolution of the density ρ(r,t) itself, the arrested state is dominated by the fluctuations of the two-point correlator ρ(r,t) ρ(r,τ + t), thus strongly depending on the actual dynamics of the system during the phase transition [4,5]. To overcome the experimental difficulty in measuring slow dynamics and the limitation of light scattering in spatial resolution as well as multiple scattering in dense and opaque solutions, we, therefore, propose here to perform an advanced low dose XPCS experiment (in USAXS mode) studying the dynamics during the LLPS of a protein solution.
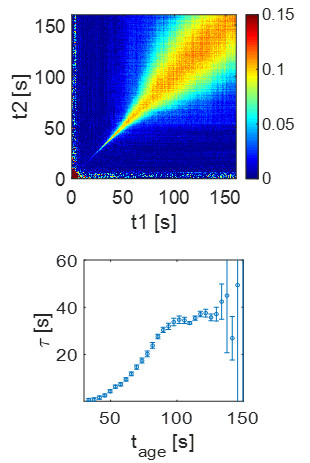
An XPCS measurement yields the intermediate scattering function which allows a detailed characterization of the system dynamics. In detail we want to measure alpha and beta relaxation times as a function of wave-vector transfer, q, and compare it to the static structure factor, S(q), for relating structure and dynamics. XPCS also provides information on Kohlrausch-Williams-Watts (KWW) exponent which can discriminate between glass-like dynamics or stress driven dynamics [4]. Measuring the non-ergodicity parameters allows us to study the degree of localization of the dynamics and facilitates comparison with the theories such as the mode-coupling theory. Furthermore, by evaluating two-time correlation (TTC) functions [6] we are able to decouple the growth kinetics and the dynamics. From TTC we want to determine the degree of heterogeneity [6] and compare it to different quenching rates.
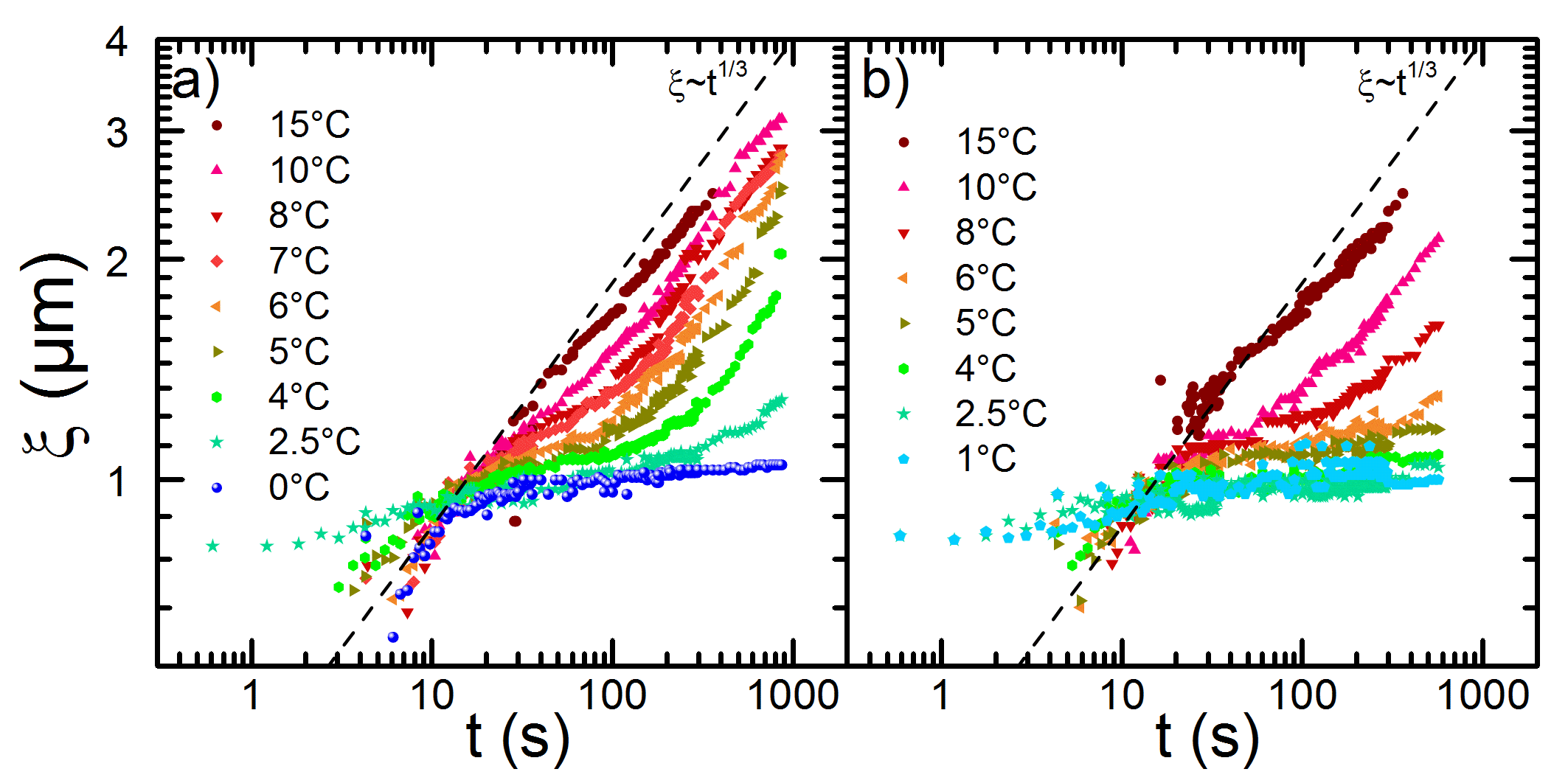
Figure 2: Plots of the characteristic length ξ as a function of time for the IgG-PEG systems. With decreasing temperatures, a transition occurs from a complete LLPS to a fully arrested state.
 We have studied the kinetics of arrested LLPS in protein
solutions using USAXS for two protein systems [7,8]. One example for
IgG-PEG system is shown in Fig. 1 with representative USAXS profiles
and the growth kinetics determined from the time dependent
characterization length. Recently, we have performed USAXS-XPCS on
these two systems to explore the dynamics of this phase transition
[9]. The newly developed
We have studied the kinetics of arrested LLPS in protein
solutions using USAXS for two protein systems [7,8]. One example for
IgG-PEG system is shown in Fig. 1 with representative USAXS profiles
and the growth kinetics determined from the time dependent
characterization length. Recently, we have performed USAXS-XPCS on
these two systems to explore the dynamics of this phase transition
[9]. The newly developed