 Proteins in solution form monodisperse
colloidal suspensions. In addition to their biological role, proteins in solution are therefore of fundamental
interest in the context of soft matter science. Proteins, however, differ in one important aspect from many
simple colloidal systems: The distribution of charges on the surface of a protein is in general
inhomogeneous. This inhomogeneous surface charge distribution can in turn be assumed to have a fundamental
biological relevance in controlling for instance aggregation phenomena and biological activity such as docking
processes. Characteristic of proteins in their native environment is the macromolecular crowding -
i.e. relatively large volume fractions being occupied by proteins - and the aqueous solvent containing salt
ions. These salt ions are crucial for the understanding of the effective interactions of proteins and the
resulting structures as well as indeed the dynamics.
Proteins in solution form monodisperse
colloidal suspensions. In addition to their biological role, proteins in solution are therefore of fundamental
interest in the context of soft matter science. Proteins, however, differ in one important aspect from many
simple colloidal systems: The distribution of charges on the surface of a protein is in general
inhomogeneous. This inhomogeneous surface charge distribution can in turn be assumed to have a fundamental
biological relevance in controlling for instance aggregation phenomena and biological activity such as docking
processes. Characteristic of proteins in their native environment is the macromolecular crowding -
i.e. relatively large volume fractions being occupied by proteins - and the aqueous solvent containing salt
ions. These salt ions are crucial for the understanding of the effective interactions of proteins and the
resulting structures as well as indeed the dynamics.
While mean-field based concepts for the description of
charge effects in colloidal suspensions have been known for some time, there are surprisingly few quantitative
studies of the effective interactions of proteins depending on their charge. Recently, new effects have been
found, most notably the reentrant condensation induced by the addition of higher-valent salts [1]. The reentrant
condensation is characterised by different regimes defined by the protein volume fraction φ and salt
concentration c: For low salt concentrations c<c* with a characteristic concentration C*(φ), the proteins
are in solution, whilst for c*<C<c** with a second characteristic concentration c**(φ), a phase
separation regime occurs, which re-enters into a solution regime for c>c** (Fig. 1). The reentrant
condensation has previously been observed for DNA or polyelectrolytes and a charge inversion theory has been
proposed to explain the observations [2]. However, this phenomenon has never been directly observed in a protein
system before. Monitoring the effect of the salt type and concentration on protein dynamics therefore promises a
deeper understanding of both the salt-protein and protein-protein interactions, including phenomena such as the
binding of salts, protein aggregation and re-dissolution. Protein aggregation by itself is a phenomenon which in
particular also has to be explored in its dynamic aspects. Controlled aggregation, namely crystallization, is
still one of the "holy grails" of protein science.
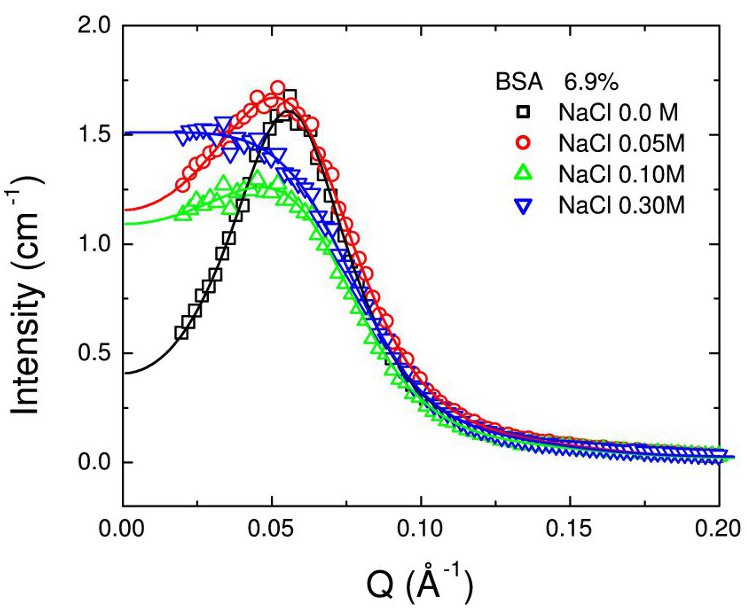 Here, we study
static and dynamic aspects of proteins in aqueous solutions containing salts. These address the issues of
crowding and of the effects of monovalent salts for the model protein Bovine Serum Albumin (BSA) [3], using a
combination of small-angle X-ray scattering (SAXS), and the quasi-elastic neutron scattering (QENS) techniques
backscattering (BS), and spin-echo (NSE). SAXS thereby accesses the static structure on protein-protein nearest
neighbour distances, whereas BS and NSE provide information on different regimes of the protein diffusion on
nanosecond time scales.
Here, we study
static and dynamic aspects of proteins in aqueous solutions containing salts. These address the issues of
crowding and of the effects of monovalent salts for the model protein Bovine Serum Albumin (BSA) [3], using a
combination of small-angle X-ray scattering (SAXS), and the quasi-elastic neutron scattering (QENS) techniques
backscattering (BS), and spin-echo (NSE). SAXS thereby accesses the static structure on protein-protein nearest
neighbour distances, whereas BS and NSE provide information on different regimes of the protein diffusion on
nanosecond time scales.
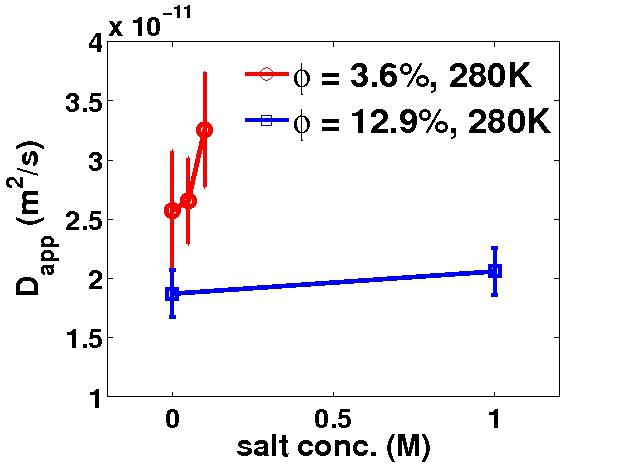
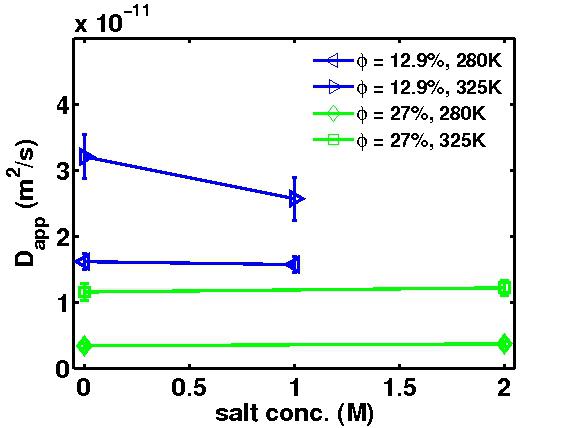 Neutron backscattering and spin-echo probe different regimes of diffusion due to
the different scattering vector ranges accessed by these techniques and different sensitivity to coherent and
incoherent scattering. The scattering vectors accessed by spin-echo are approximately commensurate with those
accessed by SAXS, whilst backscattering measures at larger vectors corresponding to intramolecular length
scales. From the backscattering and spin-echo data we find a continuously changing behaviour of the
self-diffusion of the proteins due to the excluded-volume effect. The addition of salt has little or no effect
on the apparent diffusion coefficients observed in backscattering (Fig. 3), although charge screening is assumed
to change both interaction time and coupling strength. In contrast to backscattering data, we see an increase of
diffusion upon addition of salt in neutron spin echo data (Fig. 4), whereas the dependence on protein
concentration remains qualitatively the same, i.e., a decrease of apparent diffusion upon increasing protein
concentration. In the protein concentration range thus far covered by our experiments, i.e. from approximately
4% to 27% volume fraction, our data are in agreement with a continuous decrease of the apparent diffusion
constants with the protein concentration. In contrast to the static data, our dynamic data show no distinct
value where crowding due to the excluded-volume contribution sets in.
Neutron backscattering and spin-echo probe different regimes of diffusion due to
the different scattering vector ranges accessed by these techniques and different sensitivity to coherent and
incoherent scattering. The scattering vectors accessed by spin-echo are approximately commensurate with those
accessed by SAXS, whilst backscattering measures at larger vectors corresponding to intramolecular length
scales. From the backscattering and spin-echo data we find a continuously changing behaviour of the
self-diffusion of the proteins due to the excluded-volume effect. The addition of salt has little or no effect
on the apparent diffusion coefficients observed in backscattering (Fig. 3), although charge screening is assumed
to change both interaction time and coupling strength. In contrast to backscattering data, we see an increase of
diffusion upon addition of salt in neutron spin echo data (Fig. 4), whereas the dependence on protein
concentration remains qualitatively the same, i.e., a decrease of apparent diffusion upon increasing protein
concentration. In the protein concentration range thus far covered by our experiments, i.e. from approximately
4% to 27% volume fraction, our data are in agreement with a continuous decrease of the apparent diffusion
constants with the protein concentration. In contrast to the static data, our dynamic data show no distinct
value where crowding due to the excluded-volume contribution sets in.