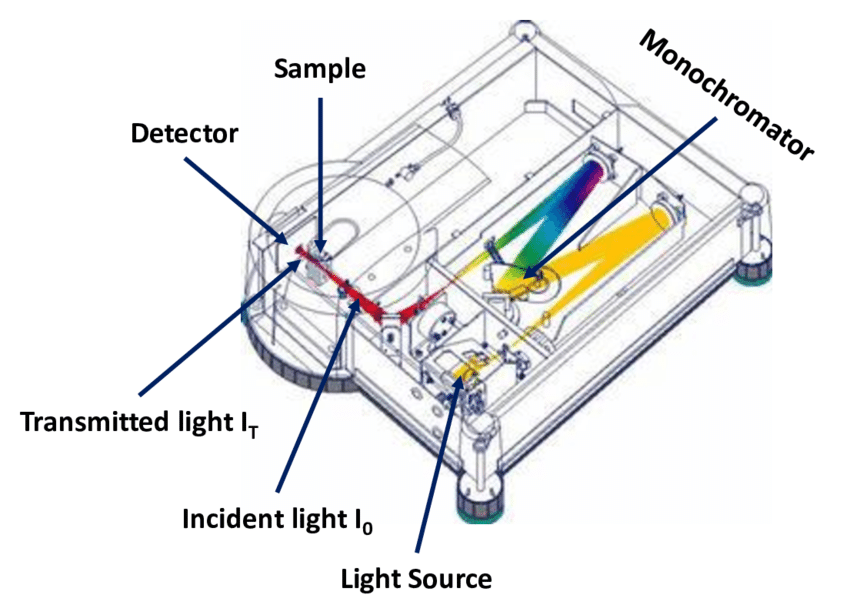
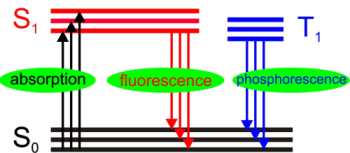
 Aromatic organic molecules exhibit a conjugated
π-electron system giving rise to delocalized electrons which on the
one hand can be excited by illumination of visible light and on the
other hand can emit light in the visible energy range. This makes
these molecules interesting for optoelectronic applications, such as
OLEDs or organic solar cells. The typical absorption and emission
processes are depicted in Fig. 1 as transitions between the singlet
electronic ground state (S0, also
called HOMO,
highest occupied molecular orbital) and the first electronic excited
state (S1, also
called LUMO,
lowest unoccupied molecular orbital).
Aromatic organic molecules exhibit a conjugated
π-electron system giving rise to delocalized electrons which on the
one hand can be excited by illumination of visible light and on the
other hand can emit light in the visible energy range. This makes
these molecules interesting for optoelectronic applications, such as
OLEDs or organic solar cells. The typical absorption and emission
processes are depicted in Fig. 1 as transitions between the singlet
electronic ground state (S0, also
called HOMO,
highest occupied molecular orbital) and the first electronic excited
state (S1, also
called LUMO,
lowest unoccupied molecular orbital).
A direct optical transition to the triplet state T1
is forbidden, which can therefore only be populated by
intersystem crossing, leading to a much longer lifetime
compared to the singlet state. The electronic relaxation
either occurs radiationless by internal conversion or
radiatively by fluorescence or phosphorescene,
respectively. Additionally, vibronic excitations take place
during either absorption or emission, depicted as sublevels of
the electronic states. Their population depends on the
electronic- vibronic coupling or, in case of aggregated
molecules called exciton-phonon coupling, described in the
next section.
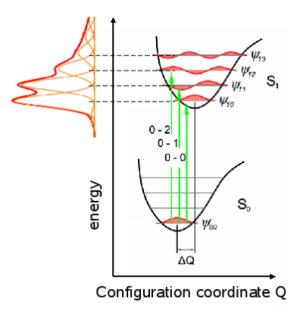 Due to a different charge
distribution between the HOMO and the LUMO electronic excitations or
relaxations lead to spatial deformations of the molecule that result
in additional vibronic excitations. This deformation is represented
by a change of the configuration coordinate Q in Fig. 2, which is in
the simplest case of a diatomic molecule the distance between the
nuclei. The bonding between the nuclei can be described by some
potential as depicted in Fig. 2 from which the vibronic states,
represented by the wavefunctions ψ, can be calculated for each
electronic state S0 and S1.
Due to a different charge
distribution between the HOMO and the LUMO electronic excitations or
relaxations lead to spatial deformations of the molecule that result
in additional vibronic excitations. This deformation is represented
by a change of the configuration coordinate Q in Fig. 2, which is in
the simplest case of a diatomic molecule the distance between the
nuclei. The bonding between the nuclei can be described by some
potential as depicted in Fig. 2 from which the vibronic states,
represented by the wavefunctions ψ, can be calculated for each
electronic state S0 and S1. Due to interactions in molecular aggregates, such as thin
films or single crystals, the optical properties are
modified. Compared to inorganic materials these interactions are
small, mainly governed by the van-der-Waals interaction. Various
effects, which are related to the structural properties of the
aggregate system, such as
Due to interactions in molecular aggregates, such as thin
films or single crystals, the optical properties are
modified. Compared to inorganic materials these interactions are
small, mainly governed by the van-der-Waals interaction. Various
effects, which are related to the structural properties of the
aggregate system, such as