Interaction of Proteins with Functional Interfaces
Introduction
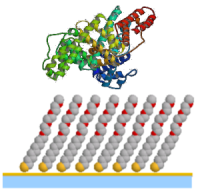 Protein interactions with interfaces
are crucial for understanding many bio-related chemical or
physical processes. For example, one of the major
difficulties with medical implants, such as hip replacement or
cardiac stents, is that the body tries to reject them. A
powerful immune response is triggered to fight the invasive
implant; therefore the ways of preventing protein from binding
to the implant are to be developed. This project aims to
develop new methods to characterize the interactions and gain
a comprehensive understanding of the phase behaviour in a
typical soft matter system comprising proteins, nanoparticles
and self-assembled monolayers (SAM) in solution. We intend to
study the structure of SAMs, stability of colloid, protein
adsorption and resistance behaviour in solution, with various
parameters such as, the size of nanoparticles, the type of
proteins, ionic strength and salt nature. The following
questions will be addressed: Protein interactions with interfaces
are crucial for understanding many bio-related chemical or
physical processes. For example, one of the major
difficulties with medical implants, such as hip replacement or
cardiac stents, is that the body tries to reject them. A
powerful immune response is triggered to fight the invasive
implant; therefore the ways of preventing protein from binding
to the implant are to be developed. This project aims to
develop new methods to characterize the interactions and gain
a comprehensive understanding of the phase behaviour in a
typical soft matter system comprising proteins, nanoparticles
and self-assembled monolayers (SAM) in solution. We intend to
study the structure of SAMs, stability of colloid, protein
adsorption and resistance behaviour in solution, with various
parameters such as, the size of nanoparticles, the type of
proteins, ionic strength and salt nature. The following
questions will be addressed:
- What is the conformation/structure adapted by thiol molecules or
adsorbed proteins at various interfaces?
- How to characterize the stability of functionalized nanoparticles and
the kinetics of aggregation by a convenient method?
- How do proteins interact with functionalized nanoparticles in
solutions? What is the key parameter determining the phase behaviour
of colloid-protein systems?
|
Protein resistance
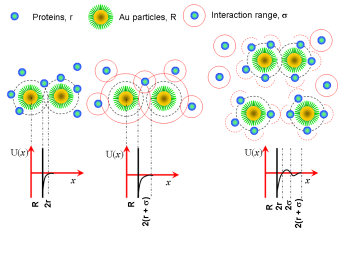 Protein resistant OEG has attracted
considerable attention due to its numerous applications in
biotechnology and medical devices, whilst protein interactions
are a key factor in determining the phase behaviour of
biological systems. The majority of studies to date have been
ex-situ and on flat surfaces. However, there are many
interesting applications of functionalized gold colloids that
may involve highly curved interfaces. For a mixture of
proteins (BSA) and gold colloids, the protein-protein
interaction changes little upon mixing with OEG SAM-decorated
gold colloids. In contrast, the colloid-colloid interaction is
found to be strongly dependent on the protein concentration
and the size of the colloid itself. Adding protein to a
colloidal solution results in an attractive depletion
interaction between functionalized gold colloids, and above a
critical protein concentration, the colloids form aggregates
and flocculate. Adding salt to such mixtures enhances the
depletion effect and decreases the critical protein
concentration. The aggregation is a reversible process. The
depletion interaction under various conditions is presented
schematically in the following figure. Protein resistant OEG has attracted
considerable attention due to its numerous applications in
biotechnology and medical devices, whilst protein interactions
are a key factor in determining the phase behaviour of
biological systems. The majority of studies to date have been
ex-situ and on flat surfaces. However, there are many
interesting applications of functionalized gold colloids that
may involve highly curved interfaces. For a mixture of
proteins (BSA) and gold colloids, the protein-protein
interaction changes little upon mixing with OEG SAM-decorated
gold colloids. In contrast, the colloid-colloid interaction is
found to be strongly dependent on the protein concentration
and the size of the colloid itself. Adding protein to a
colloidal solution results in an attractive depletion
interaction between functionalized gold colloids, and above a
critical protein concentration, the colloids form aggregates
and flocculate. Adding salt to such mixtures enhances the
depletion effect and decreases the critical protein
concentration. The aggregation is a reversible process. The
depletion interaction under various conditions is presented
schematically in the following figure.
|
Experiments
|
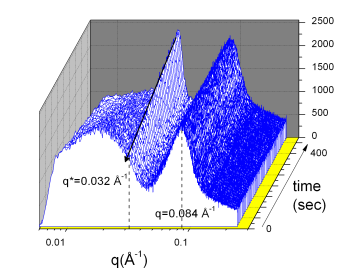 The time evolution of colloid flocculation due to
the depletion interaction can be followed as shown in the
following figure. After mixing with protein solution above a
critical concentration the real time SAXS measurements
indicate the appearance of a scattering maximum following a
short induction time, which increases its intensity with
time. However, the peak position does not change with time,
protein concentration, and addition of salt. The peak
corresponds to the distance of the nearest neighbour in the
aggregates. The time evolution of colloid flocculation due to
the depletion interaction can be followed as shown in the
following figure. After mixing with protein solution above a
critical concentration the real time SAXS measurements
indicate the appearance of a scattering maximum following a
short induction time, which increases its intensity with
time. However, the peak position does not change with time,
protein concentration, and addition of salt. The peak
corresponds to the distance of the nearest neighbour in the
aggregates.
|
|
Currently, we are working on the following issues:
- Structure of OEG SAMs on gold nanoparticles by small-angle
scattering techniques
- Interactions and phase behaviour of the mixture of protein
with SAM- coated nanoparticles
- Depletion-driven colloidal flocculation kinetics and
structure studied by real time UV-visible spectroscopy and
SAXS
- Surface modification of metal colloids, such as charge,
OEG thiol, protein absorption.
|
[1] F. Zhang, M. W. A. Skoda, R. M. J. Jacobs, R. A. Martin,
C. M. Martin and F. Schreiber. Protein interactions studied
by SAXS: effect of ionic strength and protein concentration for
BSA in aqueous solutions. J. Phys. Chem. B. 2007, 111,
251.
[2] F. Zhang, M. W. A. Skoda, R. M. J. Jacobs, S. Zorn,
R. A. Martin, C. M. Martin, G. F. Clark, G. Goerigk and F.
Schreiber. Gold nanoparticles decorated with oligo(ethylene
glycol) thiols: protein resistance and colloidal stability.
J. Phys. Chem. A. 2007, 111, 12229.
[3] F. Zhang, D. G. Dresen, M. W. A. Skoda, R. M. J. C Jacobs, S.
Zorn, R. A. Martin, C. M. Martin, G. F. Clark and F.Schreiber. Gold
nanoparticles decorated with oligo(ethylene glycol) thiols:
kinetics of colloid aggregation driven by depletion
force. Eur. Biophys. J. 2008. 37, 551
For our recent work on proteins in solution,
see list of publications.
 Protein interactions with interfaces
are crucial for understanding many bio-related chemical or
physical processes. For example, one of the major
difficulties with medical implants, such as hip replacement or
cardiac stents, is that the body tries to reject them. A
powerful immune response is triggered to fight the invasive
implant; therefore the ways of preventing protein from binding
to the implant are to be developed. This project aims to
develop new methods to characterize the interactions and gain
a comprehensive understanding of the phase behaviour in a
typical soft matter system comprising proteins, nanoparticles
and self-assembled monolayers (SAM) in solution. We intend to
study the structure of SAMs, stability of colloid, protein
adsorption and resistance behaviour in solution, with various
parameters such as, the size of nanoparticles, the type of
proteins, ionic strength and salt nature. The following
questions will be addressed:
Protein interactions with interfaces
are crucial for understanding many bio-related chemical or
physical processes. For example, one of the major
difficulties with medical implants, such as hip replacement or
cardiac stents, is that the body tries to reject them. A
powerful immune response is triggered to fight the invasive
implant; therefore the ways of preventing protein from binding
to the implant are to be developed. This project aims to
develop new methods to characterize the interactions and gain
a comprehensive understanding of the phase behaviour in a
typical soft matter system comprising proteins, nanoparticles
and self-assembled monolayers (SAM) in solution. We intend to
study the structure of SAMs, stability of colloid, protein
adsorption and resistance behaviour in solution, with various
parameters such as, the size of nanoparticles, the type of
proteins, ionic strength and salt nature. The following
questions will be addressed: Protein resistant OEG has attracted
considerable attention due to its numerous applications in
biotechnology and medical devices, whilst protein interactions
are a key factor in determining the phase behaviour of
biological systems. The majority of studies to date have been
ex-situ and on flat surfaces. However, there are many
interesting applications of functionalized gold colloids that
may involve highly curved interfaces. For a mixture of
proteins (BSA) and gold colloids, the protein-protein
interaction changes little upon mixing with OEG SAM-decorated
gold colloids. In contrast, the colloid-colloid interaction is
found to be strongly dependent on the protein concentration
and the size of the colloid itself. Adding protein to a
colloidal solution results in an attractive depletion
interaction between functionalized gold colloids, and above a
critical protein concentration, the colloids form aggregates
and flocculate. Adding salt to such mixtures enhances the
depletion effect and decreases the critical protein
concentration. The aggregation is a reversible process. The
depletion interaction under various conditions is presented
schematically in the following figure.
Protein resistant OEG has attracted
considerable attention due to its numerous applications in
biotechnology and medical devices, whilst protein interactions
are a key factor in determining the phase behaviour of
biological systems. The majority of studies to date have been
ex-situ and on flat surfaces. However, there are many
interesting applications of functionalized gold colloids that
may involve highly curved interfaces. For a mixture of
proteins (BSA) and gold colloids, the protein-protein
interaction changes little upon mixing with OEG SAM-decorated
gold colloids. In contrast, the colloid-colloid interaction is
found to be strongly dependent on the protein concentration
and the size of the colloid itself. Adding protein to a
colloidal solution results in an attractive depletion
interaction between functionalized gold colloids, and above a
critical protein concentration, the colloids form aggregates
and flocculate. Adding salt to such mixtures enhances the
depletion effect and decreases the critical protein
concentration. The aggregation is a reversible process. The
depletion interaction under various conditions is presented
schematically in the following figure.
 The time evolution of colloid flocculation due to
the depletion interaction can be followed as shown in the
following figure. After mixing with protein solution above a
critical concentration the real time SAXS measurements
indicate the appearance of a scattering maximum following a
short induction time, which increases its intensity with
time. However, the peak position does not change with time,
protein concentration, and addition of salt. The peak
corresponds to the distance of the nearest neighbour in the
aggregates.
The time evolution of colloid flocculation due to
the depletion interaction can be followed as shown in the
following figure. After mixing with protein solution above a
critical concentration the real time SAXS measurements
indicate the appearance of a scattering maximum following a
short induction time, which increases its intensity with
time. However, the peak position does not change with time,
protein concentration, and addition of salt. The peak
corresponds to the distance of the nearest neighbour in the
aggregates.