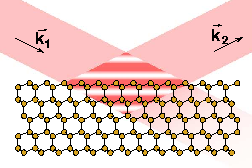
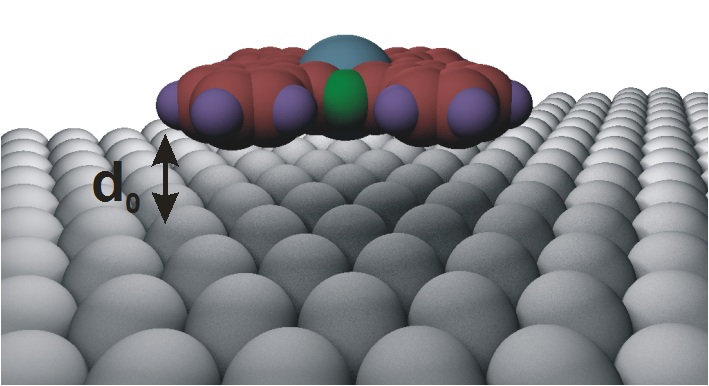
 The specific properties of
organic semiconductors - such as chemical tunability, relatively easy
and cheap processing, compatibility with flexible substrates - allow
to use these materials as active layers in different thin film
applications [1]. Among the numerous factors that influence the
overall efficiency of a device, the charge injection (respectively
extraction) process is particularly important. In this context, we
study the interaction of small conjugated molecules (COMs) with
metallic substrates. While the interface between metals and
semiconductors has been studied for many years, the resulting concepts
(Schottky-Mott theory) have to be refined for organic semiconductors
[1]. As shown by many studies in this field, the first molecular layer
on the metal is of key importance. For example, the charge
re-arrangement and the energy-level alignment at the metal-organic
interface can be related to the adsorption geometry of the molecules
[2]. The complexity of those effects, however, require sophisticated
experiments and often quantum chemical calculations [3].
The specific properties of
organic semiconductors - such as chemical tunability, relatively easy
and cheap processing, compatibility with flexible substrates - allow
to use these materials as active layers in different thin film
applications [1]. Among the numerous factors that influence the
overall efficiency of a device, the charge injection (respectively
extraction) process is particularly important. In this context, we
study the interaction of small conjugated molecules (COMs) with
metallic substrates. While the interface between metals and
semiconductors has been studied for many years, the resulting concepts
(Schottky-Mott theory) have to be refined for organic semiconductors
[1]. As shown by many studies in this field, the first molecular layer
on the metal is of key importance. For example, the charge
re-arrangement and the energy-level alignment at the metal-organic
interface can be related to the adsorption geometry of the molecules
[2]. The complexity of those effects, however, require sophisticated
experiments and often quantum chemical calculations [3].
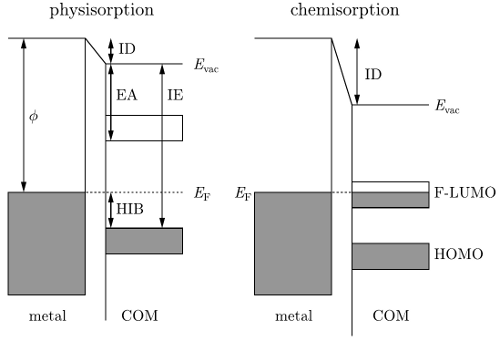 Depending on the molecule-metal combination the
interaction strength varies considerably. Two extreme scenarios can be
distinguished
Depending on the molecule-metal combination the
interaction strength varies considerably. Two extreme scenarios can be
distinguished